
As New Zealand’s infrastructure continues to age, it’s becoming more evident that effective understanding, maintenance, and treatment of sewer line odour and corrosion is critical.
A simple internet search using keywords such as “sewer line corrosion” or “H2S corrosion” can reveal alarming images of gas-phase corrosion, showcasing a significant reduction in the lifespan of collection system infrastructure. Concrete pipes are particularly vulnerable, with the rebar being the only remaining section of the top part of the pipe. While photos like these are not uncommon in the wastewater industry, it’s entirely preventable through cost-effective measures that address the problem in the collection system and offer beneficial treatment to the wastewater treatment plant downstream.

This article will address three main topics concerning sewer line odour and corrosion:
- The fundamental chemistry behind the formation of hydrogen sulphide (H2S) odour in sewer lines and its direct connection to gas-phase corrosion.
- The commonly used treatments available in the market for odour and corrosion in sewer lines.
- The advantages of using Magnesium Hydroxide as a solution for this issue.
Basic Chemistry of Sewer Odour and Corrosion
Wastewater operators often attribute sewer odour to hydrogen sulphide (H2S), but this is actually a symptom rather than the root cause of the problem. H2S is a by-product of microbiological processes that occur in the collection system. As with most aspects of wastewater treatment, it all boils down to the bugs and their maintenance. The root cause of sewer line odour is the presence of a septic or anaerobic condition that can arise under specific circumstances. The most typical cause of this condition is low flow or long detention time, such as the extremely low flow rate of sewage during late evening and early morning hours in certain collection system pump stations. In these situations, the residual oxygen in the water is depleted by chemical and microbiological activity, resulting in water with no free O2. Without free oxygen molecules, bacteria can exhibit a facultative behavior, allowing them to convert their activity from aerobic to anoxic and anaerobic, metabolizing ions like nitrate (NO3–) and sulfate (SO4-2) as sources of the O-atom necessary for their continued growth and health. These microbiologically-driven chemical reactions strip O-atoms from the ions, resulting in molecular nitrogen (N2) and hydrogen sulphide (H2S).
When microbiological reactions occur with nitrate, denitrification is the term used for the process that happens at the treatment plant. This process produces odourless and noncorrosive nitrogen gas, which actually makes up 80% of the air we breathe every day. Therefore, this reaction does not cause any odour-related issues.
However, it is the microbiological reaction with sulfate (SO4-2) that results in the formation of the unpleasant rotten-egg odour of H2S. In addition to the formation of H2S, there are other microbiological processes that occur under anaerobic conditions that generate acids known as volatile fatty acids. As these acids are produced, the pH of the wastewater can decrease, leading to a state referred to as “going septic”. A good example of this is a restaurant grease trap, which may be covered with a thick layer of grease, causing the pH of the water beneath the cap to drop as low as 4 or 5.
Another instance where this happens is in fire sprinkler lines, which are filled with fresh city drinking water. However, when they are flushed, they release a strong H2S odour due to the microbiological conversion of sulfate into sulphide. These lines also become black with corrosion product, which is essentially what used to be the pipe wall! This process of odour and corrosion is similar to what occurs in certain sewer lines where long detention times are common.
Hydrogen sulphide is also a weak acid in water that exists in two possible forms: H2S and HS–.
It is noteworthy that when the pH of the water is acidic (pH < 7), H2S is the predominant species, which is the volatile and unpleasant-smelling gas that needs to be eliminated. Conversely, when the water is basic (pH > 7), HS- is the most dominant species, which is nonvolatile and deserves to be kept in mind for our discussion.
Intriguingly, when sewage is trapped within a force main, the SO4-2 🡪 H2S process can be catalytic, indicating that sulfate can be transformed into H2S by microorganisms. Later, within the pipe, H2S can be converted back into sulfate and then back into H2S, and so on. However, unfortunately, the reactions that reverse the conversion of H2S to SO4-2 can lead to accelerated corrosion within the system in most instances.
To put it simply, in collection systems, odour and corrosion are closely related through the chemical reactions of sulfate and H2S, mainly driven by microorganisms. H2S is not the root cause of the problem, but rather a symptom. The longer the wastewater stays in the pipe, the lower the oxygen level, and the more anaerobic conditions allow microorganisms to produce acids, including H2S. The acidic environment makes H2S more volatile, allowing it to escape into the gas-phase, causing corrosion. The reaction between H2S and sulfate in enclosed sections of the sewer line, such as force mains, can be catalytic, leading to a cycle of conversion between H2S and sulfate that causes accelerated corrosion. In collection systems, the phrase “if it stinks, it corrodes” summarises the relationship between odour and corrosion.
Odour and Corrosion Control Treatments
The industry employs various methods to tackle odour complaints and infrastructure damage caused by corrosion in collection systems and lift stations. Some methods solely target odour, such as the use of scrubbers to filter H2S or sealing manhole lids. Although these approaches may effectively eliminate odour complaints, they do not tackle the ongoing corrosion within the system.
Of the chemical treatment methods, the most dominant in the industry fall into the following categories:
Denitrification
There are several products available in the market that make use of denitrification, which is the microbiologically driven reduction of nitrate into nitrogen gas that is odour-free and non-corrosive. These products typically contain nitrate salts, such as calcium nitrate, and are added to collection systems to provide an alternative oxygen source. This directs the bacteria in the biomass to preferentially choose NO3– instead of SO4-2, resulting in a significant reduction in odour. This treatment approach is considered relatively environmentally friendly and cost-effective, and has been widely accepted in the industry.
However, one potential issue with this approach is that it requires a higher dose of nitrate than stoichiometric to achieve odour reduction performance. As a result, the overall nitrogen level in the system may increase, which could be a concern for treatment plants that have recently received new regulations on the amount of nitrogen they can discharge. Additionally, this treatment approach promotes the growth of biomass in the system, which could reduce the pumping capacity of the system and require periodic mechanical cleaning (also known as “pigging”).
Shock Treatment
This method typically involves the introduction of a large amount of a highly alkaline chemical, such as Caustic Soda, or a strong oxidizing agent, such as Bleach, in a process known as slug dosing, to raise the pH or biocide concentration to extreme levels. This results in a short-term benefit by causing the detachment of large amounts of biomass from the pipe. However, without continued treatment, the biomass and corresponding odour quickly return, requiring another dose. This rapid recovery of microorganisms demonstrates their incredible resilience. This is evident in the drinking water distribution system, where biofilms have been found to survive and grow despite the continuous presence of chlorine or chloramine. For instance, when a city worker accidentally bumps a drinking water pipe, downstream from the location, brown water may appear. This is surprising since clean drinking water is pumped into a clean pipe. However, microorganisms are remarkably resilient!
Precipitation
Another frequently utilized method for controlling odours involves causing H2S to precipitate into a mineral that becomes part of the suspended solids transported within the system. This is achieved through the use of precipitating agents, with iron salts like ferric chloride or ferrous sulfate being the most commonly used, leading to the formation of iron sulphide precipitates. The primary advantages of this treatment include the relatively low cost of iron salts and their rapid reaction with H2S, resulting in a quick reduction of odour. However, these acidic products can pose significant risks to operators handling them, lower the pH of the wastewater flowing to the treatment plant, and increase the amount of suspended solids entering the plant, which can be a disadvantage.
Oxygenation
As mentioned earlier, the fundamental cause of odour and corrosion is the lack of oxygen in the collection system, leading to anaerobic conditions. Therefore, various methods have been developed to supply the system with oxygen to maintain a dissolved oxygen residual. The key advantage of this approach is that it does not require the purchase of chemicals, except for liquid oxygen. However, the challenge lies in the capital and maintenance costs of oxygenation systems, as well as the need to regulate the oxygen dose accurately. Excessive gaseous oxygen can cause air release valves to trip and clog or result in water hammer and pump cavitation. Additionally, many treatment plants rely on the presence of acids, such as volatile fatty acids (VFAs), produced by anaerobic microorganisms as a readily available food source for the treatment process. Maintaining a dissolved oxygen residual in the collection system eliminates the formation of VFAs.
pH Adjustment
Another method for controlling gas-phase odour and corrosion caused by H2S is to prevent its escape from the water-phase by adjusting the pH of the water towards the alkaline direction. As mentioned earlier, hydrogen sulphide is a weak acid that is always in equilibrium with its counter-anion, HS–. Raising the pH in the collection system causes the equilibrium to shift in favour of HS–, which is a non-volatile ion that does not leave the water-phase. This approach completely mitigates odour and gas-phase corrosion. Furthermore, the added pH and alkalinity typically have a positive impact on the downstream treatment plant.
Magnesium Hydroxide for pH Control
There are several chemical methods available to increase water pH, but they often come with negative consequences. Caustic soda (NaOH) can cause pH spikes or loss when under-fed and is hazardous to handle. Soda ash (Na2CO3) is typically fed as a powder and adds two moles of sodium per dissolved molecule, increasing sodium and total dissolved solids (TDS) levels in the effluent. Lime (Ca(OH)2) can cause calcium carbonate scale formation, restricting flow and minimizing pumping capacity. Moreover, it is also hazardous and cumbersome to handle and feed, making it less desirable for feed into a collection system.
In contrast, magnesium hydroxide (Mg(OH)2) dissolves relatively slowly in water and provides a long-lasting buffer, making it a better alternative. It is completely nonhazardous, allowing operators to handle and feed it with confidence. Additionally, it is beneficial for aquatic and terrestrial life as the Mg2+ ion is the core element of chlorophyll, which drives photosynthesis. A 60% Mg(OH)2 product can release a larger amount of hydroxide (OH–) into the water from a single dose than any other liquid alkaline additive.
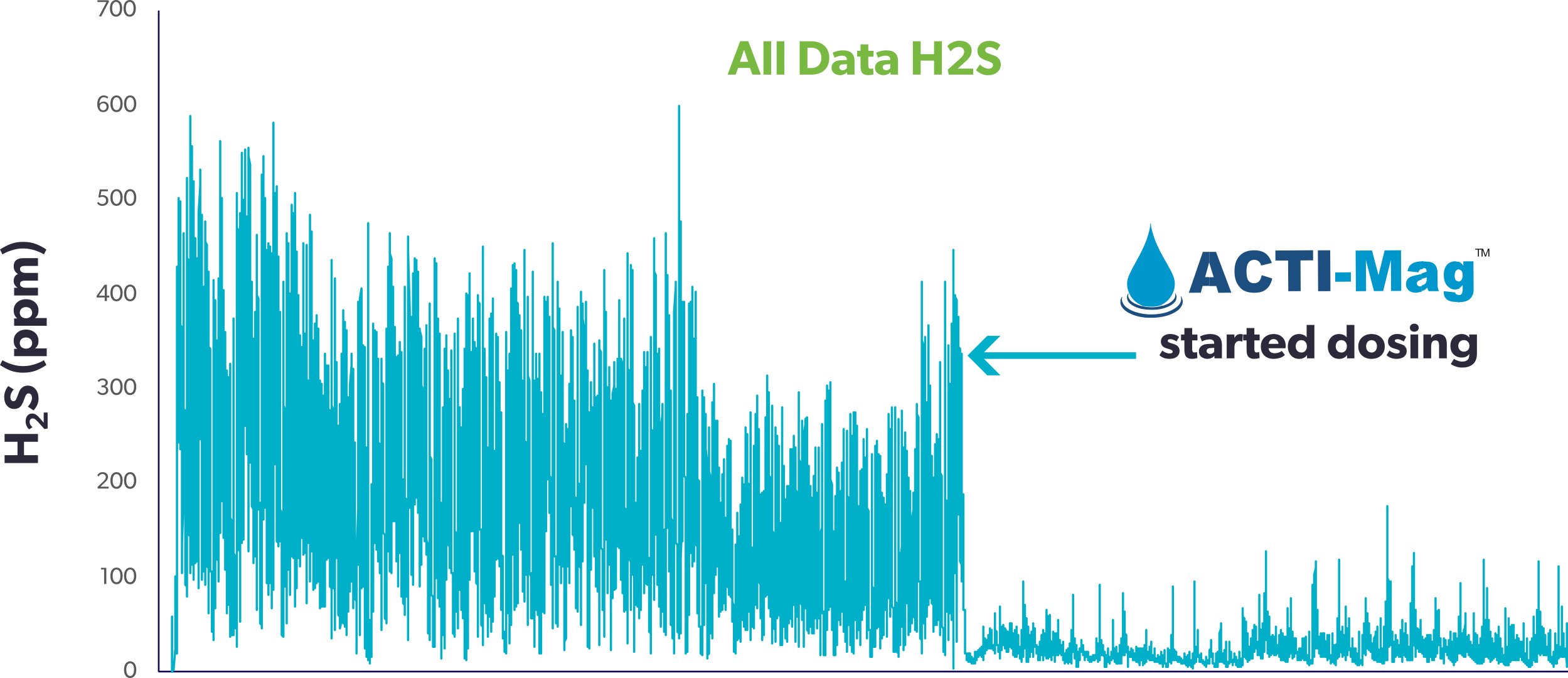
The data presented in Figure 1 shows the effectiveness of magnesium hydroxide in mitigating H2S in a real-world application for odour control in New Zealand. The product used, ACTI-Mag, is a 60% Mg(OH)2 product. The dosing was done at a pump station approximately 7 km from the pump station and resulted in a significant reduction in H2S levels. This treatment also effectively controlled odour and corrosion in the rising main and reduced the accumulation of fats, oils, and greases (FOGs) in the pump station station. Pump stations downstream of the site also benefited from the added alkalinity, reducing H2S levels from around 70ppm to 5ppm.
However, a drawback of using magnesium hydroxide is that it is a slurried product that needs to be agitated in storage to ensure reliable feed. To overcome this concern, magnesium hydroxide feed systems have been designed with optimised agitated storage tanks and simplified, robust chemical metering systems. Although the cost of installing a proper magnesium hydroxide feed system may be higher than other alkaline products, the reduced chemical usage results in overall cost savings.
Compared to other alkaline additives, ACTI-Mag has a greater neutralizing value per pound, resulting in a 40% reduction in chemical usage in comparison to caustic soda. Moreover, it is much safer for operators to handle and provides more nourishment for the microorganisms being sustained. As a result, it is the most economical alternative for managing hydrogen sulphide gas (H2S) in sewer systems.
Conclusion
The main cause of odour and corrosion in sewer lines is the occurrence of low flow conditions that lead to an anaerobic environment in the system. During such periods, microorganisms produce acids, including hydrogen sulphide, which lowers the pH and allows volatile acid gases to escape, causing rapid gas-phase corrosion. Introducing an alkaline additive to the low-flow section of the collection system can convert these acids into nonvolatile counter anions, thereby preventing odour or corrosion. Magnesium hydroxide is the preferred choice of alkali due to its ability to remove biomass and increase pump capacity in the collection system.
One of the key advantages of this approach is that the increased pH and alkalinity in the collection system also benefits the downstream treatment plant, unlike some other odour control treatments that may have adverse effects downstream. The boost in influent pH and alkalinity helps to stabilize these parameters as the wastewater flows through the plant, particularly in the context of increasing nitrogen removal requirements. As a result, the plant can achieve significant cost savings by reducing or eliminating the use of hazardous chemicals like caustic soda, as well as energy savings from reducing aeration blower operation.
Moreover, magnesium hydroxide is environmentally-friendly, safe, and nonhazardous, and it has a lower usage rate than any other alkaline additive, making it the most effective choice for odour and corrosion control in the collection system.
